methyl acetate formation
What is the equation for formation of methyl acetate. Another method of production is the esterification of methanol and acetic acid in the presence of a strong acid.
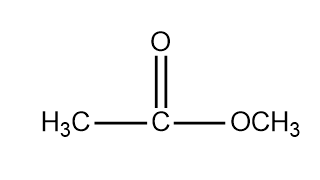
Methyl Ethanoate Is An Ester Write Its Structural Class 11 Chemistry Cbse
Methyl acetate can be further hydrogenated to ethanol which an important chemical and a fuel additive 1.

. 7-Methoxy-1-naphthyl methyl acetate 7-Methoxy-naphtha-1-yl-acetic acid 0225 mol was dissolved in a mixture of 700 ml benzene and 300 ml HOAc and PbOAc 4 0225 mol added. Methyl acetate MA is an important VOC that originates from degradation of methyl tert-butyl ether MTBE tert amyl ether TAME and ethyl tert-butyl ether ETBE. Methyl chloroacetate C3H5ClO2 CID 7295 - structure chemical names physical and chemical properties classification patents literature biological activities safetyhazardstoxicity information supplier lists and more.
C 3 H 7 O 2 Molecular weight. Afterward the methoxide inter- mediate attacks a carbonyl group of the anhydride forming methyl acetate and the methylcarbonate ion Figure 2 R3. It is a colourless volatile liquid with a pleasant odour and fleeting fruity like the taste.
Information on this page. C 3 H 6 O 2. The mixture heated to 6070 C refluxed 30 minutes cooled concentrated extracted with 200 ml CH 2 Cl 2.
500 ml Diethyl ether was added causing a creamy white precipitate which was. A variety of solid base catalysts were prepared by loading Cs2O over SiO2 or Al2O3 as the support materials. Show transcribed image text.
Methyl acetate MA is produced by various methods such as the esterification of acetic acid the reaction between methanol and acetic anhydride the reaction between peroxycarboxylic acid and acetone and wood pyrolysis. It is formed from the condensation of acetic acid and methanol. Use condensed structural formulas to write the equation for the formation of methyl acetate.
The Pd- catalyzed reductive carbonylation of methyl acetate with CO and H2 affords acetaldehyde. The molecular or chemical formula of Methyl acetate is C 3 H 6 O 2. Methyl acetate is produced industrially by liquid phase reaction of acetic acid and methanol in presence of an acid catalyst.
The net reaction is the formation of acetaldehyde from MeOH CO and H2P4. When acetic acid and methanol it forms methyl acetate and water Ch3COOHCH3OH--CH3C2H3O2H20. The formation of the meoac with the yield of 18 over nickel-copper chlorides on the bac-a grade carbon support is shown to be facilitated by the optimal combination of the characteristics of the porous structure mesopores with an average diameter of 7 nm and the surface acidity of the catalyst.
Methanol is burned with acetic acid in the presence of sulfuric acid to make methyl acetate. Methyl acetate can be made in a variety of ways some of them are listed below Carbonylation is one method that is utilized in industry. 1819 Methylcarbonate cannot serve as methyl.
Also the direct use of MA in coatings metal industries plastics automobile industries printing solvents and dyes causes the accumulation of MA in the atmosphere through evaporation 8 9 10. The vapor-phase aldol condensation of methyl acetate MeOAC with formaldehyde HCHO to form methyl acrylate was investigated over solid base catalysts. Acetic acid methyl ester.
Carbon monoxide substrates are brought together in these reactions. To produce methyl acetate methanol is heated alongside acetic acid in the presence of sulfuric acid. Handling Storage Distribution Hazards Toxicity.
15 of meoac yield over nicl 2 cucl 2. The properties of the solid base catalysts were characterized by XRD BET and CO2-TPD. C 3 H 6 O 2.
Empirical formula Hills system for organic substances. Sulfuric acid is a common catalyst also used in this reaction. At 57 C it is a colourless and flammable liquid which is used as a solvent for oils and many resins.
Methyl formate 109 is converted into AcOH under CO pressure in the presence of Lil and Pd OAc2 95. Methyl acetate is produced industrially via the carbonylation of methanol as a byproduct of the production of acetic acidmethyl acetate also arises by esterification of acetic acid with methanol in the presence of strong acids such as sulfuric acid this production process is famous because of eastman kodaks intensified process using a reactive. Copy Sheet of paper on top of another sheet.
See the answer See the answer See the answer done loading. Use condensed structural formulas to write the equation for the formation of methyl acetate. What is methyl acetate used for.
This problem has been solved. Switch to calorie-based units.
Ethyl Acetate Molecule Of The Month March 2003 Html Version

Reactions Considered In The Formation Of Methyl Acetate By Alkylation Download Scientific Diagram

Chemical Derivatization Of Acetate Using Methyl Chloroformate Mcf Download Scientific Diagram

Methyl Acetate Metac 99 5 Solvents Wacker Chemie Ag

Chemical Derivatization Of Acetate Using Methyl Chloroformate Mcf Download Scientific Diagram
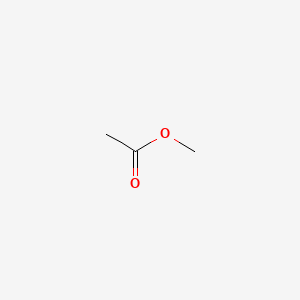
Methyl Acetate Ch3cooch3 Pubchem
Ethyl Acetate Cas 141 78 6 109623
Synthesis Of Phenylmethoxycarbonylamino Methyl Acetate Via Ester Formation From Carboxylic Acid Chemsink
What Smell Can I Get When Mixing Methanol With Acetic Ethanoic Acid Quora

Fatty Acid Methyl Ester Production Via Ferric Sulfate Catalyzed Interesterification Sciencedirect

Reactions Considered In The Formation Of Methyl Acetate By Alkylation Download Scientific Diagram
File Synthesis Of Methyl Acetate Svg Wikimedia Commons

Methyl Acetate An Overview Sciencedirect Topics
Ethyl Acetate Molecule Of The Month March 2003 Html Version
What Is Reaction Of Methanol And Ethanoic Acid Quora

Methyl Acetate An Overview Sciencedirect Topics

Datei Synthesis Of Methyl Acetate Svg Wikipedia
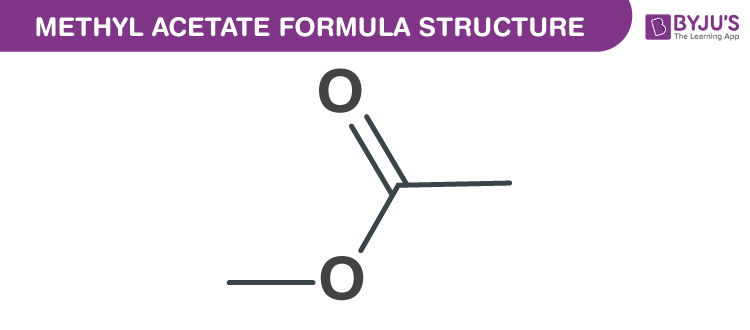
Methyl Acetate Formula Chemical Formula Structure Properties And Uses

Comments
Post a Comment